Examples Of Alkenes And Characteristics In Chemistry
Alkenes are chemical elements with double bonds or carbon double bonds. Its general formula is CnH2n.
Alkene Definition
Alkenes are hydrocarbons that contain at least one carbon-carbon double bond (C=C). The general molecular formula for alkenes is CnH2n, where “n” is the number of carbon atoms.
These atomic structures differ from alkanes by having a greater amount of hydrocarbons, which causes the hydrogen atoms to be reduced in their formulas.
The characteristics of alkenes do not allow their resistance to solid bonds, that is, they are hydrocarbons but to the extent that they create new carbon bonds, they subtract hydrogen atoms, so they become insoluble and cannot create bonds with elements such as nitrogen.
Key Characteristics Of Alkenes:
-
Double Bond
Alkenes have a carbon-carbon double bond, consisting of one sigma bond (σ) and one pi bond (π).
The pi-bond is formed by the overlap of parallel p-orbitals on the carbon atoms.
-
Naming
The parent chain is selected to include the carbon atoms involved in the double-bond, and the suffix “-ene” is added to indicate the presence of a double bond.
-
Isomerism
Alkenes exhibit both structural and geometric isomerism.
Structural isomers have the same molecular formula but differ in the arrangement of atoms.
Geometric isomers, also known as cis-trans isomers, arise due to the restricted rotation around the carbon-carbon double bond.
-
Reactivity
Alkenes are more reactive than alkanes due to the presence of the pi bond.
-
Physical Properties
(a): They have lower boiling points than alkanes.
(b): Similar molecular weight because the double bond creates a region of electron density that affects the intermolecular forces
-
Polymerization
Alkenes can undergo polymerization reactions to form polymers. For Example: Ethene (C2H4) can polymerize to form polyethylene.
Alkanes vs Alkenes: What’s The Difference?
Both are chemical elements made up of carbon and hydrogen.
Alkanes, for their part, are composed of hydrogen atoms while Alkenes in their structure subtract soluble atoms.
Alkenes are called alkanes without hydrogen, since their nuclear components remain the same, governed by carbon.
By strengthening the carbon bonds above the hydrogen bonds, they are able to structure themselves as hydrocarbon chemical elements.
5 Examples Of Alkenes In Chemistry
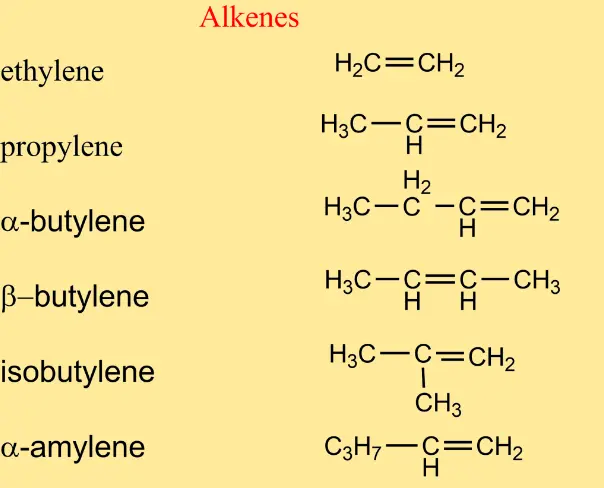
- Ethene or ethylene: Made up of two carbon atoms, it is one of the most famous alkenes used industrially for the manufacture of polyethylene. Ethylene is also a natural plant hormone that accelerates plant growth in stable temperatures, and is also used in fruits for ripening.
- Propene or propylene: like all alkenes, it has a double carbon bond. Alcohols are obtained from it.
- Butene or butylene: It is obtained from petroleum derivatives and is useful for the manufacture of flammable gases.
- Cyclopentene: It is obtained in a liquid state, similar to petroleum. It is used for the disintegration of plastics.
- Polyethylene: It is used to create plastics due to its malleability .
Also READ:
- Different Types Of Alkenes And Examples in Chemistry
- What is a chemical reaction? Types, Characteristics, Parts And Examples
- Chemical Phenomena with Examples in Chemistry
- 17 Laboratory Safety Rules and precautions for chemistry labs
- Acids and Bases examples & Properties
- Difference between absorption and adsorption in tabular form



