What is a chemical reaction? Types, Characteristics, Parts And Examples
We explain what a chemical reaction is, the types that exist, its speed and other characteristics. In addition, physical and chemical changes.
What is a chemical reaction?
Chemical reactions (also called chemical changes or chemical phenomena ) are thermodynamic processes of matter transformation . These reactions involve two or more substances , called reagents or reactants, which change significantly in the process, being able to consume or release energy.
This means that every chemical reaction subjects the matter to a profound transformation, altering its molecular structure and composition (as opposed to physical changes that only affect its form or state of aggregation). Chemical changes generally produce new substances , different from what we had at the beginning.
Chemical reactions are very common and can occur spontaneously, under various conditions in nature , and also in the controlled environment of a laboratory, due to the manipulation of the human being.
Many of the materials we use daily are obtained industrially, from simpler substances combined by one or several reactions.
Physical and chemical changes in matter
The physical changes of matter are those that alter its shape without changing its composition , that is, without modifying the type of substance in question. For example, by boiling water we can convert a liquid into a gas, but the resulting vapor is still composed of hydrogen and oxygen atoms. The same if we freeze it: it takes a solid form, but is chemically the same.
On the other hand, chemical changes alter the distribution and bonds of atoms in matter , making them recombine differently and thus obtaining substances different from the initial ones, although always in the same proportion, since matter cannot be created or Destroy easily, just recombine.
This is what happens if we react water (H 2 O) and potassium (K), we will obtain two new substances: potassium hydroxide (KOH) and hydrogen (H 2 ), in a reaction that normally releases a lot of energy.
Characteristics of a chemical reaction
Chemical reactions are generally irreversible , since they usually involve the loss or gain of energy. That is to say that they involve the formation or destruction of atomic bonds between the molecules of the reagents.
Therefore, matter is deeply transformed, although sometimes this recomposition cannot be seen with the naked eye. Even so, the proportions of the reagents can be measured, which stoichiometry deals with.
On the other hand, chemical reactions yield certain products depending on the nature of the reagents, but also on the conditions in which the reaction occurs. Therefore, it is possible to control the result obtained by adding catalysts : third substances that act solely as controllers of the reaction without fully participating in it.
Types of chemical reaction
Chemical reactions are classified, first and foremost, according to the type of reagents that compose them. Therefore, we will distinguish between organic and inorganic chemical reactions, each classified independently:
Inorganic reactions . They involve inorganic compounds , and they can be of four different types:
- Synthesis or addition reactions . Two reagents combine with each other to result in a different substance.
- Analysis or decomposition reactions . A complex substance reacts with another and unfolds into two of its simplest components.
- Displacement reactions . A compound or element occupies the place of another within a larger or more complex compound, replacing it and leaving it free.
- Double substitution reactions . Two reagents exchange compounds or chemical elements simultaneously.
Organic reactions . They involve organic compounds, such as those linked to life . They depend on the type of organic compound for classification, since each functional group has a range of specific reactions: alkanes, alkenes, alcohols , ketones, aldehydes, etc.
On the other hand, chemical reactions can also be endothermic (when they consume energy) or exothermic (when they release energy), depending on the nature of their reagents.
Parts of the chemical reaction
Reactant or Reagent
 Reagents or reactants are those substances that develop an interaction with others in a chemical reaction producing other substances with different characteristics, properties and conformation, which are called reaction products or products.
Reagents or reactants are those substances that develop an interaction with others in a chemical reaction producing other substances with different characteristics, properties and conformation, which are called reaction products or products.
Reagents are chemical compounds that are classified taking into account different variables, such as reactivity in chemical reactions, physicochemical properties and the characteristics of the use of the reagent.
This classification is taken for granted in the reagent container and is based on the treatment that has been provided for both its purity and its richness, these factors determine the chemical utility that will be given, taking into account the accuracy, the precision and the absolute error that can happen in the chemical operation to be carried out.
The reagents or reactants can be classified under the following names:
- PA: These are used in analytical applications.
- PB: They are destined to the area of biochemistry.
- DC: Its object of study is the applications of clinical analysis.
- QP: Its acronym means chemically pure and is generally used in the laboratory.
Products

The products of a chemical reaction are defined as the chemicals that result from the separation and rearrangement of reactants. These appear on the right side of the equation in the chemical reaction. In general, the products are much more stable molecules than the reactants.
Something important to keep in mind is that the distinction between the products and the reactants is not very clear because these reactions are carried out in an equilibrium way, this means that there is a back and forth process between the products and the reactants. .
The end result is that some of the reactants can combine to form products, however these chemicals can react again to end up forming the reactants again. When this kind of reaction reaches equilibrium, reactants and products coexist together and remain in constant variation between the two states.
Symbology
 Different types of symbols are used to denote the functions within the chemical reaction, depending on their appearance each symbol has a specific meaning.
Different types of symbols are used to denote the functions within the chemical reaction, depending on their appearance each symbol has a specific meaning.
The symbols that appear in a chemical reaction are the following:
- Right Arrow (→): This indicates a direct reaction.
- Left Arrow (←): Specifies a reverse reaction.
- Reversible arrows: Express a reversible reaction.
- Triangle (▲): Indicates heat.
- Up arrow (↑): Expresses the formation of a gas.
- Down arrow (↓): This symbol indicates that there is the formation of a solid that is in a state of precipitation.
- CC: It expresses the need for direct current to carry out the reaction.
- 200 ° – 400 °: It expresses the temperature that is needed for the reaction to take place.
- Letters (g, s, l, ac): They indicate the state of the reaction substances.
Importance of chemical reactions
Chemical reactions are fundamental to the existence of the world as we know it and understand it today. The changes that matter undergoes in natural conditions and that often throws valuable materials is just one example, since the greatest evidence of the importance of chemical reactions is life itself, in all its expressions.
The existence of living beings of all kinds is only possible thanks to the ability of matter to react, which allowed the first cell forms of life to exchange energy with their environment through metabolic pathways, that is, through chemical reaction sequences that threw more useful energy from which they consumed.
For example, in our daily life breathing is composed of multiple chemical reactions. They are also present in the photosynthesis of plants .
-
Speed of a chemical reaction
Chemical reactions require a stipulated time to happen, which varies depending on the nature of the reagents and the environment in which the reaction occurs.
For example, the addition of heat usually accelerates certain reactions , by incorporating freely available energy into the reagents. On the other hand, it is also possible to use catalysts : substances that accompany the reaction without altering its results, but which allow to shorten the reaction time considerably.
Other factors that alter the speed of a reaction are the atmospheric pressure, the concentration of the reagents, and even the state of aggregation in which they are found: solids usually react more slowly than liquids or gases, although it will always depend on it, Again, the nature and reactivity of each substance.
-
How is a chemical reaction represented?
The chemical reactions are represented by chemical equations , that is, formulas in which the participant reagents and the results obtained are described, often indicating certain conditions of the reaction, such as the presence of energy, the creation of ions (electrically charged particles ), etc.
The first chemical equation in history was written in 1615 by Jean Beguin , in one of the first treatises on chemistry , the Tyrocinium Chymicum. Today they are common teaching, and thanks to them we can more easily visualize what is happening in a given reaction.
Examples of chemical reaction

Some simple chemical reactions are:
- Ethylene + Bromo = 1,2 dibromoethane (pesticide)
C 2 H 4 + Br 2 à C 2 H 4 Br 2
- Methane + oxygen + energy = water + carbon dioxide
CH 4 + O 2 + E à 2H 2 O + CO 2
- Hydrogen peroxide (+ Time) = water + oxygen
2H 2 O 2 à 2H 2 O + O 2
- Caustic soda + hydrochloric acid = Sodium chloride (salt) + water
NaOH + HCl à NaCl + H 2 O
- Iron + oxygen + water = ferric oxide + water
4Fe + 3O 2 + H 2 O à 2Fe 2 O 3 + H 2 O
Examples of chemical reactions in everyday life
Reaction between acids and bases
If you’ve ever had stomach acid, then you’ve experienced such a reaction. Sodium bicarbonate is widely used to calm stomach acid, since it is a base, which reacts with stomach acids and releases carbon dioxide.
Cry for the onion
Have you ever wondered: Why does the onion make me cry when cutting it? This vegetable contains molecules of amino acids sulfoxides. When you cut an onion, the cell walls are broken, thus releasing sulfoxides and enzymes that turn it into a sulfenic acid that irritates the eyes.
Digestion
Our digestion is a chemical process, in which enzymes intervene that transform nutrients from complex molecules to simpler ones.
Hydrocarbon combustion
We experience this chemical process every time we turn on the gas stove. If this process is done with little oxygen, then it generates carbon monoxide, which is poisonous.
Coloring in old books
Each page of an old book turns yellow, this is because the cellulose of the paper decomposes, this gives it a yellowish tone and a very peculiar smell, similar to that of vanilla. The molecules in charge of this process are called lignin or vanillin.
Examples of chemical reactions that take place around you every day

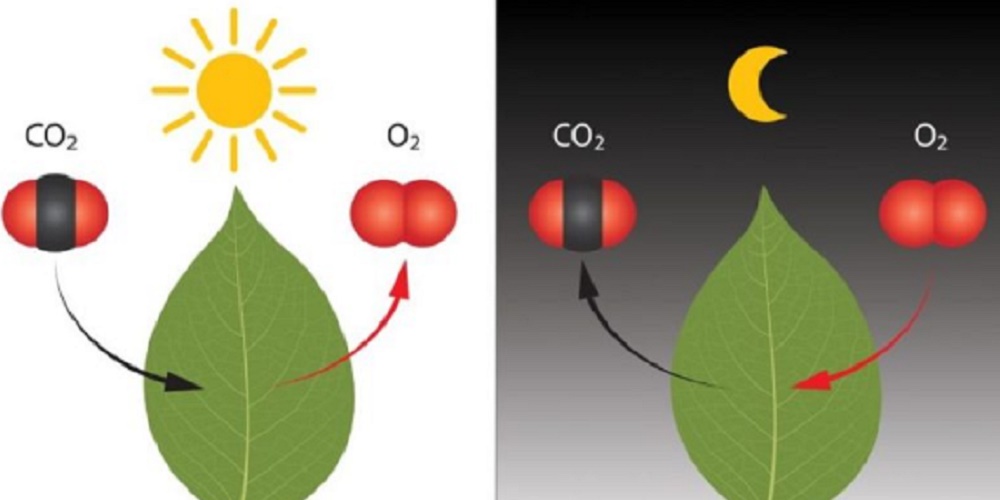
Photosynthesis

Plants apply a chemical reaction called photosynthesis to convert carbon dioxide and water into food (glucose) and oxygen. It is one of the most common daily chemical reactions and also one of the most important, as this is the way plants produce food for themselves and animals and convert carbon dioxide into oxygen. The equation for the reaction is:
Aerobic cellular respiration
Aerobic cellular respiration is the opposite process of photosynthesis in which energy molecules combine with the oxygen we breathe to release the energy needed by our cells, in addition to carbon dioxide and water. The energy used by cells is chemical energy in the form of ATP, or adenosine triphosphate.
Here is the general equation for aerobic cellular respiration:
C 6 H 12 O 6 + 6O 2 → 6CO 2 + 6H 2 O + energy (36 ATPs)

Anaerobic respiration is a set of chemical reactions that allows cells to obtain energy from complex molecules without oxygen. Your muscle cells perform anaerobic respiration whenever the oxygen delivered to them is depleted, such as during intense or prolonged exercise. Anaerobic respiration by yeast and bacteria is harnessed for fermentation to produce ethanol, carbon dioxide, and other chemicals that make cheese, wine, beer, yogurt, bread, and many other common products.
The general chemical equation for a form of anaerobic respiration is:
C 6 H 12 O 6 → 2C 2 H 5 OH + 2CO 2 + energy
Combustion

Every time you light a match, burn a candle, light a fire, or light a grill, you see the combustion reaction. Combustion combines energetic molecules with oxygen to produce carbon dioxide and water.
For example, the equation for the propane combustion reaction , found in gas grills and some fireplaces, is:
C 3 H 8 + 5O 2 → 4H 2 O + 3CO 2 + energy
Mold

Over time, iron develops a red, flaky coating called rust. This is an example of an oxidation reaction . Other everyday examples include the formation of verdigris on copper and silver stains.
Here is the chemical equation for the oxidation of iron:
Fe + O 2 + H 2 O → Fe 2 O 3 . XH 2 O
Metathesis chemical reaction

If you combine vinegar and bicarbonate of soda by a chemical volcano or milk with baking powder in a recipe, you will experience a double displacement , or metathesis reaction (plus a few others). The ingredients are recombined to produce carbon dioxide gas and water. The carbon dioxide forms bubbles in the volcano and helps rise in baked goods .
These reactions seem simple, but in practice they often consist of several steps. Here is the general chemical equation for the reaction between baking soda and vinegar:
HC 2 H 3 O 2 (aq) + NaHCO 3 (aq) → NaCl 2 H 3 O 2 (aq) + H 2 O () + CO 2 (g)
Electrochemistry chemical reaction

Batteries use electrochemical or redox reactions to convert chemical energy into electrical energy. Spontaneous redox reactions occur in galvanic cells , while non-spontaneous chemical reactions take place in electrolytic cells .
Acid-base chemical reaction

Whenever you combine an acid (for example, vinegar, lemon juice, sulfuric acid , or muriatic acid ) with a base (for example, baking soda , soap, ammonia, or acetone), you are performing an acid-base reaction . These reactions neutralize the acid and base to give salt and water.
Sodium chloride is not the only salt that can be formed. For example, here is the chemical equation for an acid-base reaction that produces potassium chloride, a substitute for common table salt:
HCl + KOH → KCl + H 2 O
Soap and detergents chemical reaction

Cleaning soaps and detergents through chemical reactions . Soap emulsifies dirt, which means oil stains stick to the soap so they can be lifted off with water. Detergents act as surfactants, lowering the surface tension of water so it can interact with oils, insulate them, and rinse them away.
Kitchen

Cooking uses heat to cause chemical changes in food. For example, when an egg is hard boiled, the hydrogen sulfide produced by heating the egg white can react with the iron in the egg yolk to form a grayish-green ring around the yolk . When meat or baked goods brown in color, the Maillard reaction between amino acids and sugars produces a brown color and a desirable flavor.
Examples of instantaneous chemical reactions
These types of chemical reactions are also known as rapid reactions and are carried out immediately after the reactants come together. Most of these reactions involve ionic species, which is why they are known as ionic reactions.
Examples of rapid reactions
- Metabolization of sugar in our body, to obtain energy.
- Neutralization of an acid using a base.
- Combustion of a methane gas.
- Glycerin combustion using potassium permanganate.
- Decomposition of hydrogen peroxide using potassium iodide.




