Difference between absorption and adsorption in tabular form
We elaborate that what is the difference between absorption and adsorption with table. Chemistry is the study of matter, as well as its properties, how substances combine to generate another substance, or how they separate, resulting in a third substance / substances. All these activities are carried out with the help of intermolecular forces.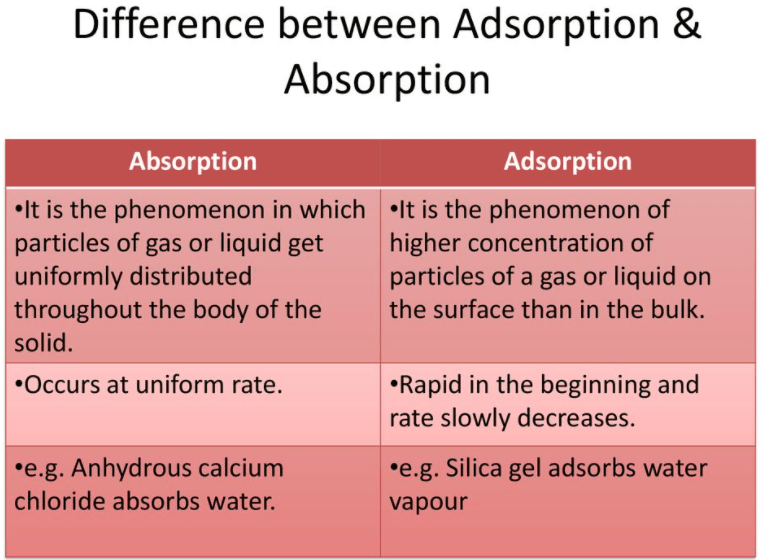
Intermolecular forces or IMF are the forces that arbitrate the interaction between different or equal molecules, atoms or ions, through the act of attraction or repulsion. Examples of attractive intermolecular forces are hydrogen bonds.
There are mainly four types of intermolecular forces, ionic bonds, hydrogen bonds, Van der Waals dipole-dipole interactions, and Van der Waals scattering interactions. Intermolecular forces are of great importance as they lead to physical differences between similar molecules, their boiling and melting points, when the molecules change state as solid to liquid, liquid to gaseous or liquid to gaseous state.
Absorption, in simple words, means the act of absorbing or taking, while on the other hand, adsorption is the adhesive act of holding the molecules of a liquid or a gaseous state on a surface.
The main difference between absorption and adsorption is that the former means taking or accepting molecules, atoms or ions through a chemical reaction, while the latter denotes the ability of all solid substances to attract molecules from the liquid or gaseous state that is in contact with the surface.
Comparison table between absorption and adsorption (in tabular form)
Adsorption absorption comparison parameter
| Sense | In absorption, a substance is incorporated into the physical structure of another substance. | In the case of adsorption, a substance or energy is attracted to the surface of another substance. |
| Examples | A paper soaked in water absorbs the water due to the absorption process. | Activated carbon in a gas mask is an example of adsorption. |
| Components | Two components are involved in absorption, such as the absorbent and the absorbent. | In adsorption, two components involved are the adsorbate and the adsorbent. |
| Molecules | In absorption, the molecules enter most of the phase. | In the case of adsorption, the molecules adhere to the surface of the phase. |
| Temperature | In absorption, the process is not affected by temperature. | On the other hand, in the case of adsorption, the process is affected by temperature. |
What is absorption?
Absorption is a massive process of accepting molecules or other particles as ions or atoms, through a chemical or molecular reaction, as a result of which a new substance is formed or takes place.
The absorbent material is known as absorbate, it remains intact in other substances, which is known as absorbent due to the presence of space within the substance but they do not have any chemical reaction with each other. Once the substance or absorbent is absorbed into another substance, it cannot be easily separated. In absorption, the substance is uniformly distributed throughout the liquid or gaseous state. The liquid or gas particles are evenly distributed throughout the solid body.
They are used commercially in the cooling system, cold storage and refrigerants.
For example:
- Water vapors absorbed by anhydrous CaCl2.
- NH3 is absorbed in water to form NH4OH.
There are two types of absorption processes:
- Chemical absorption: Chemical absorption occurs due to a chemical reaction between the substances being absorbed and the absorbent medium. It also depends on the concentration of the reagents.
- Physical absorption: In physical absorption, the capacity of the solvent increases following Henry’s law, and the solvent is regenerated by reducing the pressure.
What is adsorption?
Adsorption is the process of keeping molecules or other particles from the liquid or gaseous state intact with the surface. The substance that is absorbed is called adsorbate and the solid on which the process occurs is called adsorbent. It involves attraction or retention of molecules on the surface. Adsorption implies uneven distribution in bulk and on the surface of the molecule.
For example, hydrogen (H2), nitrogen (N2), and oxygen (O2) are adsorbed on the surface of carbon. Absorption and adsorption involve two different mechanisms.
For example:
- Water vapor adsorbed by silica gel.
- NH3 is adsorbed by charcoal.
The two types of adsorption processes are:
- Physical adsorption: Physical adsorption is also known as physisorption and is due to the weak Van der Waals force between the adsorbate and the adsorbent.
- Chemical adsorption: Chemical adsorption is also known as chemisorption and is caused by strong chemical forces and the bonding between adsorbate and adsorbent.
The adsorption of gas on a solid is the result of a spontaneous exothermic reaction. The amount of heat that is released when a unit of gas is adsorbed is called the heat of adsorption.
Main differences between absorption and adsorption
- Absorption is a process by which atoms, molecules, or ions go into mass. Whereas, adsorption is the accumulation of molecular species on the surface, not in bulk.
- Absorption is an endothermic process, while adsorption is an exothermic process.
- Absorption is a volume phenomenon, on the contrary, adsorption is a surface phenomenon.
- The absorption process remains the same throughout the material, while adsorption is a process that is determined by the concentration of the substances.
- The absorption process occurs at the same speed or uniformly, on the other hand, the adsorption process is fast at first and eventually slows down.
Final Thought
Absorption and adsorption are homophones, which means that they may appear similar, but they have different meanings, as well as their application. Both are part of Chemistry, Physics and Biology, since in the three branches of science they play an important role but they are different.
Absorption is the process of absorbing or assimilating or incorporating molecules or other particles due to a chemical or molecular process, while, on the other hand, when molecules, ions or atoms of the liquid or gaseous state are attracted or connected to the surface of a molecule, the act of keeping them glued to the surface is known as adsorption.