What is Biological Composition in science? Definition and examples
Cells are the basic unit of life. All living organisms are made up of one or more cells. As will be discussed later, the human body is made up of millions of cells. In order to know the problems that occur in tumours, it is necessary to understand the regularity of normal cell activity. First, let’s discuss the composition and function of cells. biological composition
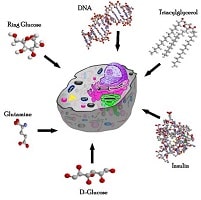 Let us first discuss the composition of the cells. All cells, regardless of function and location, share common attributes and processes. Surprisingly, almost all cells are composed of four basic molecules. Shown above is a cell around which these basic molecules are listed. biological composition
Let us first discuss the composition of the cells. All cells, regardless of function and location, share common attributes and processes. Surprisingly, almost all cells are composed of four basic molecules. Shown above is a cell around which these basic molecules are listed. biological composition
Because these basic molecules exist in organisms, we call them biomolecules. The structure and function of these basic molecules will be discussed one by one below.
- Carbohydrate
- protein
- Lipid
- Nucleic acid
- Biomolecular complex
Carbohydrate biological composition

The first type of biomolecule we will discuss is the carbohydrates. Such molecules are composed of basic elements such as carbon (C), hydrogen (H), and oxygen (O). We usually call this type of molecule sugar. Carbohydrate molecules can be very small or very large. Like all other biomolecules, carbohydrate molecules are often strung together by smaller units to form long chains. Just like the beading on the wrist, it is made up of a string of beads that are very long. We refer to this structural unit (Imagery “beads”) is called a monomer (Monomer), the long chain monomer is called polymer (Polymer).
For example, the sugar (lactose) and sugar (sucrose) contained in milk are all carbohydrates. Shown below is the molecular structure of glucose. Glucose is a monomeric sugar that is the main source of energy for our body. biological composition
Glucose biological composition
Glycogen
Protein
Like carbohydrates, proteins are also made up of smaller units. Such protein-constituting monomers are called amino acids. There are about twenty different amino acids. There is an amino acid called glutamine, and its structure is shown in the following figure: biological composition
Glycine
- The composition of many special structures (such as hair, nails, muscles, protein proteins) involved in the body is the main structural component of cells and membranes.
- Participate in the translocation of transmembrane substances. For example, glucose enters cells from the blood. In discussing the “drug resistance” of tumour cells to chemotherapy drugs, we will discuss the function of proteins in this area.
- Acts as a biocatalyst. This is a large class of proteins called enzymes that speed up the chemical reactions required for cellular activity. For example, many enzymes are involved in the breakdown of food so that nutrients can be used by the body. biological composition
- The exchange of information between cells plays a very important role in maintaining the structure and function of cells and organs. Proteins are often responsible for contact between adjacent cells and between the cells and their local environment. For example, the interaction between cells and cells allows our skin cells to be tightly connected together. The interaction between cells is in turn dependent on proteins in closely connected neighbouring cells. Cell interactions is altered metastatic (metastatic necessary condition).
- Proteins can control the activity of a cell, for example, it can determine whether a cell divides. Cancer cells are always defective on such proteins. We will discuss these proteins in detail in the section “Regulation of cell division”.
- Many hormones that alter the behaviour of cells or organs are also composed of proteins. The following shows the insulin (insulin), which is a small protein hormones, glucose play a role in regulating body obtained from the blood.
Lipids (Lipid) This term refers to a large class of biomolecules including fats, oils, wax, steroids (Steroid) hormone (hormones). Although they differ in structure, distribution, and function in and between cells, all lipids have the following common features and are therefore classified into one class.
- Insoluble in water, both hydrophobic (hydrophobic).
- Like carbohydrates, it consists mainly of carbon, hydrogen, and oxygen. biological composition
The hydrophobic nature of lipids makes it multifunctional in biological systems. Fat is a good form of energy storage. Oils and waxes are used to form skin protective films to prevent infection. Certain lipids, such as steroid hormones, are important regulators of cellular activity. We will mention this again in the “information flow” section of the cell. Such as estrogen ( Estrogen such as steroid hormones), and cancer of the female reproductive system related. The treatment will be discussed in detail in the relevant chapters.
Triacylglycerol
The main function of lipids is to form bio membranes. The cells are surrounded by a thin layer of lipid. The lipid layer is made of a special type of lipid, has both hydrophobic (Hydrophobic) have hydrophilic ( Hydrophilic ). The “hydrophilic end” of such molecules faces the inner environment filled with water and the multi-water environment outside the cell. Between these two layers is a hydrophobic region. Cell membranes are rich in proteins and other lipids such as cholesterol .

Most chemicals cannot pass through the lipid bilayer. Water and some small molecules can pass freely through the cell membrane, but other molecules must be actively transported by protein channels in the cell membrane. The cell membrane also contains a combination of biomolecules that have not yet been explained. The figure above shows that proteins can bind to carbohydrates to form glycoproteins. As mentioned before, glycoproteins are important for cell-to-cell communication. Changes in the amount and type of glycoproteins are common in cancer. Similarly, lipids can be combined with carbohydrates to form sugar esters.
Nucleic acid

All information for controlling and building cells is stored in nucleic acid molecules. biological composition
A nucleic acid ( Nucleic acid ) divided into two categories, i.e. deoxyribonucleic acid ( the DNA ) and ribonucleic acid ( an RNA ). Both types of molecules are polymers. Like the above-mentioned carbohydrates and proteins, they consist of subunits such as monomers. The monomers that make up the nucleic acid are called nucleotides. All kinds of nucleotides are often represented by acronyms, namely A, C, G, T, U. As with all of the mall thescribed above, the monomers constituting the DNA are similar but not identical to each other. One of the differences between DNA and RNA is that the nucleotides used to construct the polymer differ. DNA contains A, C, G, and T, while RNA contains A, C, G, and U.
Deoxyribonucleic acid (DNA)
DNA is composed of two nucleotide long chains (multimers) that are twisted to each other to form a double helix as shown in the following figure. These distorted molecules are specifically arranged and the specific nucleotides are always matched in pairs. For example, a nucleotide containing adenylate (A) is always paired with a nucleotide containing thymine (T); likewise, guanine (G) is always paired with cytosine (C). If you look closely at the image below, you will find that the paired nucleotides are arranged in the middle of the double helix. The polymer chains that form DNA can be very long, and each DNA molecule can contain millions of nucleotides. The figure below shows a portion of the short chain in the DNA of the double-stranded structure. 1
DNA
Ribonucleic acid
RNA is similar to DNA in many ways. It is also a multimer of nucleotides that carries genetic information. In addition to the difference in chemical structure between RNA and DNA, they also have the following important functional differences.
- In the nucleus, RNA is copied from DNA, and much of the information in the RNA has been transferred to the cell fluid.
- RNA is a functional form of information stored in DNA.
- RNA is single-stranded, not double-stranded.
For cells, the information stored in DNA is like the design blueprint used by architects. The specific replication of RNA allows cells to use only the pages needed on the “blueprint” at a time. Copying the right RNA at the right time is very important for the cell. In cancer, the replication and regulation of RNA is abnormal. Just as the “reading the picture” error causes problems in the built house, RNA replication errors can lead to changes in the way cells behave, leading to the emergence of cancer. This important topic will be further elaborated in the “Gene Function” section. First, we will discuss more complex forms of biomolecules and then introduce several important functional structures of eukaryotic cells.
Biomolecular complex
At present, the main biomolecules that have been introduced are as follows: biological composition
- Carbohydrate
- Lipid
- protein
- Nucleic acid
The above biomolecules can be combined to perform certain functions of the cells and constitute certain structures of the cells. For example, in the “Lipid” section, the first thing we see is the schematic of the cell membrane below.

In addition to the bilayer membrane structure composed of a special type of lipid, the cell membrane also contains a large amount of protein and sugar. As shown, proteins and sugars often combine to form glycoproteins; sugars can also bind to lipids to form glycolipids.
In the formation and diagnosis of cancer, most of the proteins with important effects are glycoproteins. For example, the test for the diagnosis of prostate cancer is to detect the presence of glycoproteins in the blood with or without prostate-specific antigens. The diagnosis of ovarian cancer is to detect the presence of a glycoprotein called CA-125 . Where CA stands for “tumor correlation.” biological composition
More information about the CA-125 trial.
Many kinds of proteins and biomolecules often bind together to form some functional structures within the cell. Later, we will also discuss more complexes collectively called organelles.
to sum up biological composition
All organisms, including the cells that make up the human body, are made up of several types of biomolecules. Here are the four main categories:
- Carbohydrate
- Carbohydrates are composed of carbon (C), hydrogen (H), and oxygen (O) molecules.
- Sugar is a common carbohydrate.
- The main functions of carbohydrates in cells are:
- Primary energy
- Provide structure
- communication
- Cell attachment
- Resist and remove foreign matter
- protein
- Proteins are made up of amino acids.
- In living organisms, proteins have the following functions:
- Hair, muscle, nail, cell composition and cell membrane structure
- Cell transport
- Biocatalyst or enzyme
- Maintain cell contact
- Control cell activity
- Signal transmission through hormones
- Ester
- It includes many types of biomolecules such as fats, oils, waxes and steroid hormones.
- The ester is insoluble in water (hydrophobic) and consists mainly of carbon (C), hydrogen (H), and oxygen (O).
- In vivo, esters have the following functions:
- Biofilm formation
- Fat can be stored as an energy source
- Grease and wax provide protection by covering areas (skins, ears) that may be invaded by microorganisms
- Steroid hormones regulate cellular activity by altering gene expression
- Nucleic acid
- All the information needed to control and construct cells is stored in these molecules.
- Nucleic acids consist of nucleotides abbreviated as A, C, G, T, and U.
- There are two main forms of nucleic acid: deoxyribonucleic acid (DNA) and ribonucleic acid (RNA):
- DNA
- DNA has a double-stranded structure and consists of nucleotides A, C, G, and T.
- The DNA is located in the nucleus.
- DNA is a form of storage of genetic information.
- RNA biological composition
- RNA is generally single stranded and consists of nucleotides A, G, C, and U.
- RNA is copied from DNA and is a functional form of DNA information
- RNA is collectively expressed in cells and within it, and mRNA is transported into the cytosol.
- DNA
More biomolecules can be formed from a combination of these four molecules. For example, many proteins change due to the attachment of carbohydrates, and the final product is called glycoprotein. biological composition



