What is a chemical compound?
We explain what a chemical compound is, what types exist and the chemical composition of water. In addition, the chemical elements.
-
What is a chemical compound?
A chemical compound is any substance formed by the union of two or more types of chemical elements , that is, by atoms of two or more different types, linked together by chemical bonds of some kind. That is, substances that can be decomposed by chemical reactions in their constituent chemical elements, will always be considered compounds .
By incorporating more atoms and molecules , chemicals grow in complexity and size. They can gather a few different elements or hundreds of them in an impressive framework.
For example, biatomic substances such as carbon dioxide (CO 2 ) or water (H 2 O) are chemical compounds . So are other more complex ones such as sulfuric acid (H 2 SO 4 ) or glucose (C 6 H 12 O 6 ), or even inexpressible macromolecules in a simple chemical formula , such as a human DNA molecule .
Despite being more or less complex agglomerations of elements, chemical compounds have a stable set of physical and chemical properties : water is always identical to itself, as is sulfuric acid .
On the other hand, a seemingly minor change in the configuration of its constituent atoms can produce radical changes in these properties , or it can produce completely new substances through a chemical reaction.
-
Types of chemical compounds
Chemical compounds can be classified according to two different criteria, which are:
According to the method of bonding their atoms . Depending on what type of link exists between the constituent elements of a compound, we can talk about:
- Molecules , joined by covalent bonds ( electron loan).
- Ions , joined by electromagnetic links and endowed with positive or negative charge.
- Intermetallic compounds , joined by metallic bonds , which usually occur between atoms of the metallic type.
- Complexes , which hold their long structures together through coordinated covalent bonds.
According to the nature of its composition . Depending on the type of atoms that integrate them, we can talk about:
- Organic compounds . Those who have carbon as a base element, around which others are structured. They are the fundamental compounds, moreover, for the chemistry of life. They can be aliphatic, aromatic, heterocyclic, organometallic or polymers .
- Inorganic compounds . Those that have nothing to do with life, and that are grouped together in different ways according to their fundamental properties in nature. They are also classified as:
- Basic oxides , in which a metal reacts with oxygen.
- Acid oxides , bonds between oxygen and a non-metallic element .
- Hydrides , hydrogen bonds with metallic or nonmetallic elements.
- Hydrazides , hydrogen bonds with non-metallic elements that dissolve in water form an acid.
- Hydroxides , or bases, which are the result of diluting a basic oxide in water, are characterized by their hydroxyl functional group (OH-).
- Oxacids , compounds obtained from the reaction between an acid oxide and water, are regularly known as acids.
- Binary salts , formed by the union of a hydrazide and a hydroxide.
- Oxisales , formed by the union of an oxacid and a hydroxide.
-
Daily examples of chemical compounds

It is not difficult to find everyday examples of chemical compounds. Just take a look at the kitchen: chemical compounds are water (H 2 O), sugar or sucrose (C 12 H 22 O 11 ), salt (NaCl), oil (glycerol and three carboxylate radicals) or vinegar , which is a dilution of acetic acid (C 2 H 4 O 2 ).
It is not different, although at much higher levels of complexity, what happens with butter, cheese, milk or wine .
-
Chemical elements and chemical compounds
Chemical compounds are agglutinations of chemical elements of different complexity. That is to say that the chemical elements are the minimum pieces of matter , which cannot be broken down into smaller pieces.
That is, water is a compound that contains oxygen and hydrogen, but these last two, even if they are in a pure state in nature (which would be as diatomic gases: O 2 and H 2 ), contain only molecules of atoms identical to each other.
-
Water chemical composition
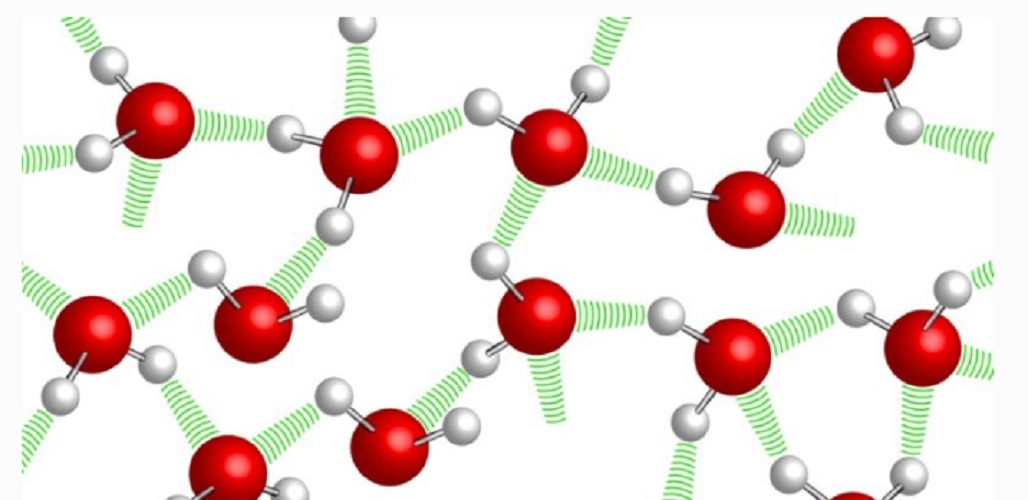
As it denotes its chemical formula (H 2 O), despite being a simple substance, the water is a chemical compound consisting of two types of elements: hydrogen (H) and oxygen (O) , in a fixed ratio and determined in each of its molecules: two atoms of the first for each of the second.
These atoms are linked by covalent bonds, which give the molecule great stability. In addition, they grant dipole properties that allow the formation of bridges between the hydrogen atoms of a water molecule and the others (hydrogen bridges).





