Difference between base and alkali in tabular form
we explain that a base and alkali are basic ionic salts or alkaline earth metals or simply alkali metals from which alkali is a base that dissolves in water or water-soluble bases. on the other hand, the base is any substance slippery to the touch, tastes bitter, changes the color of indicators in a water solution.
The compound, ion, or element which can donate a pair of electrons to the other species can act as a base. the base does not dissolve in water the basic.
The basic Differences Between Alkali and Base are that Alkali is compounds that are dissolved in water and form hydroxide. baking soda is a common example while the base is a substance that is used to neutralize an acid. NaOH and KaOH are common examples.
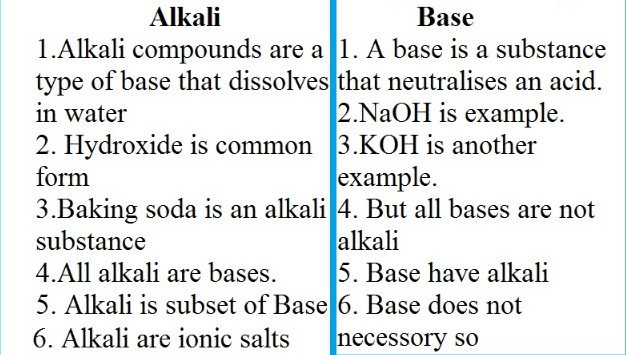
Difference Between Alkali and Base in tabular Form
| Alkali | Base |
| Alkali is Hydroxides of alkali metals and alkaline earth metals | it may be an ion or element which can donate a pair of electrons to the other species |
| alkali is used to refer to metals found in group 1 of the periodic table | the base is a compound that consists of hydroxide ions or lone electron pairs. |
| Bases that dissolve in water is known as alkali | do not dissolve in water |
| Belongs to group 1 metals | the chemical category is any ionic or covalent |
| E.g. Sodium hydroxide (NaOH) | E.g. NaOH and KOH furnish OH- which can donate a pair of electrons. |
Read Also: What is base?
You May Also Read:



