What is a chemical element?
We explain what a chemical element is, its characteristics and various examples. In addition, the periodic table and chemical compounds.
-
What is a chemical element?
A chemical element is each of the fundamental forms of matter . It always presents itself as atoms of the same and unique type, and therefore cannot be broken down into simpler substances yet.
That is, when we talk about a chemical element or simply an element, we refer to a certain type of known isotomes, which differ from others in their nature and their fundamental properties . This is usually expressed by different symbols for each one.
The atoms of the different chemical elements are, then, different from each other, but equal to themselves. That is to say that one atom of hydrogen (H) and another of oxygen (O), for example, differ from each other in, first of all, the number of protons that have a nucleus (that is, their atomic number ) : hydrogen has only one (1), while oxygen has eight (8).
On the other hand, all hydrogen atoms are identical to each other, and all oxygen atoms are identical as well. The exception to this principle is atomic isotopes: variants of each element that have different properties , since some are more massive or more stable than others, but not different enough to constitute a different element.
For example, hydrogen has three isotopes: light hydrogen ( 1 1 H), the most abundant; deuterium ( 2 1 H), which has an additional neutron; and tritium (T), which has two additional neutrons.
When a chemical reaction occurs between two or more substances, it is their chemical elements that are exchanged, constituting new atomic bonds and thus forming new forms of matter. That is to say that everything that exists is composed of combinations of the same elements .
-
Chemical elements in the Periodic Table
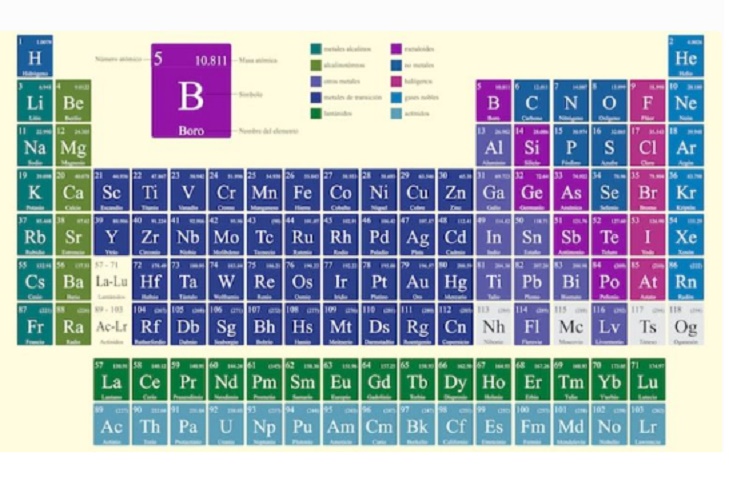
The Periodic Table of the Elements is a way of representing all known chemical elements in an orderly manner (expressed by their chemical symbols). They are grouped based on their common electronic and chemical properties, ranging from those with the lowest atomic number to those with the highest.
This table was presented in its first version by Dmitri Mendeléyev in 1869 . Since then it has been expanded, updated and improved, until obtaining its most current versions.
The Periodic Table distributes the elements in groups (horizontal) and periods (vertical), thus forming sets of elements called categories, such as: Metals (divided into Alkalines, Alkaline-earths, Lanthanides, Actinides, Transition metals and other metals), metalloids and no metals (divided into Halogens, Noble Gases and Other nonmetals).
The IUPAC version of the Periodic Table can be seen here .
-
Examples of chemical elements

Some of the best known chemical elements are:
- Hydrogen (H)
- Carbon (C)
- Oxygen (O)
- Nitrogen (N)
- Phosphorus (P)
- Sulfur (S)
- Aluminum (Al)
- Iron (faith)
- Chlorine (Cl)
- Iodine (I)
- Sodium (Na)
- Calcium (Ca)
- Potassium (K)
- Mercury (Hg)
- Silver (Ag)
- Gold (Au)
- Copper (Cu)
- Uranium (U)
- Argon (Ar)
- Zinc (Zn)
- Helium (He)
- Neon (Ne)
- Lead (Pb)
-
How many elements are there?
Currently 118 different elements are known , each described in the periodic table. However, some of them are synthetic, that is, artificial: they do not exist in nature but only in the laboratories of mankind.
Thus, the latest chemical technologies have allowed us to find up to 129 different elements , many of which do not exist beyond a short period of time in very specific conditions of a specialized laboratory.
-
Chemical compound
Chemical compounds are understood as the forms of matter that arise from the combination of the different atomic elements . Thus, they can be biatomic molecules , such as carbon dioxide (CO 2 ) or complex compounds with lots of different atoms, such as organic macromolecules (for example, DNA).
The truth is that all compounds are substances that can be reduced, given the appropriate chemical reactions, in their constituent elements, becoming increasingly simple until they reach the monoatomic or elementary substances. For example, water (H 2 O) can be decomposed by electrolysis into hydrogen and oxygen molecules, both in the form of gas.




