What is anaerobic respiration?
We explain what anaerobic or anaerobic respiration in biology is, what types exist and examples of regions where it occurs.
-
What is anaerobic respiration?
In biology, the metabolic process of oxidoreduction of sugars is called anaerobic respiration or anaerobic respiration . That is to say that in this process glucose is oxidized for energy , without the presence of oxygen. That is, a process of cellular respiration in which oxygen molecules do not intervene.
Anaerobic respiration differs from aerobic or aerobic respiration since the latter requires oxygen to process the sugar molecules. On the contrary, anaerobic uses another type of chemical elements or even more complex organic molecules , through an electron transport chain .
Nor should it be confused with fermentation , since it does not involve the electron transport chain. However, both processes have in common that they occur in the absence of oxygen.
This type of cellular respiration is exclusive of certain prokaryotic organisms ( bacteria or archaea), especially those that live in conditions of little or no presence of oxygen. However, in many cases it can also be a secondary process, say emergency, given the unexpected shortage of this element in the environment.
-
Types of Anaerobic breathing
Anaerobic respiration can be classified according to the type of chemical element used to replace oxygen , that is, as an electron receptor during the metabolic process. Thus, there can be many types of processes of this nature, but the main and most common are:
- Anaerobic respiration through nitrates . In this case the microorganisms consume nitrates (NO 3 – ) to reduce them to nitrites (NO 2 – ) by incorporating electrons. However, given that nitrites are usually toxic to most life forms , it is much more common for the final product of this process to go further, to biatomic nitrogen (N 2 ), which is an inert gas. This process is known as denitrification.
- Anaerobic respiration by sulfates . Similar to the previous case, but with sulfur derivatives (SO 4 2- ), it is a much rarer case, belonging to totally anaerobic bacteria, while the previous case may occur as an alternative to the momentary oxygen shortage. Sulfur radicals (S 2- ) are subproduced in this sulfate reduction process .
- Anaerobic respiration using carbon dioxide . Some groups of arches producing methane gas (CH 4 ) consume carbon dioxide (CO 2 ) for use as an electron receptor. Of this nature are the microorganisms that inhabit the digestive tract of ruminants, for example, where other microorganisms supply them with the hydrogen they require for the process.
- Anaerobic respiration by iron ions . The latter case is common among certain bacteria, capable of consuming ferric ions (Fe 3+ ), reducing them to ferrous ions (Fe 2+ ), since these types of iron molecules are very common in the earth’s crust. It is what happens at the bottom of the swamps, where important sediments of iron occur due to bacterial action.
-
Examples of anaerobic respiration

Examples of this type of process are common in the prokaryotic world, especially in the most inhospitable regions of the planet , but not for that reason devoid of life. Such regions are:
- The intestines of upper animals.
- The seabed and the abyssal cracks.
- The geothermal locks where magma sprouts to the bottom of the sea.
- Geysers, hot springs and other forms of geothermal outbreak .
- The swamps and clayey waters , full of organic matter and low oxygen.
The following table shows different electron acceptors, their products and some examples of microorganisms that perform such processes. The first example describes aerobic respiration and the others anaerobic.
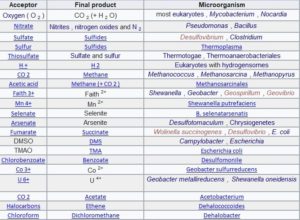
Use of nitrate as an electron acceptor
Many bacteria anaerobic contain the enzymes nitrate reductases that catalyze the reduction of nitrate to nitrite
However, the resulting product (nitrite) is very toxic so some species of Pseudomonas and Bacillus can reduce nitrate beyond the nitrite level, down to molecular nitrogen
The end result, nitrogen, is an inert and non-toxic gas . This process is known as denitrification, which, if it occurs in the soil, is considered harmful to agriculture since it causes the loss of nitrates, necessary for plant growth.
Nitrate-reducing bacteria are facultative anaerobes since the use of nitrates and nitrites as electron acceptors are alternative processes that these bacteria can use to grow in the absence of oxygen. In its presence, even though nitrate is present, respiration proceeds entirely through the aerobic electron transport chain.
Use of sulfate as an electron acceptor
The use of sulfate as an electron acceptor is a rare skill, restricted to the genus Desulfovibrio and some species of Clostridium . All of these bacteria are strictly anaerobic , so reducing sulfate is not an alternative to their metabolism, as is reducing nitrate. The reaction is as follows:
Use of carbon dioxide as an electron acceptor
A small group of strictly anaerobic prokaryotes , the methane-producing archaea , use carbon dioxide as an electron acceptor; the reduction gives rise to methane (CH 4 ). The simplest case is the oxidation of molecular hydrogen , an energy-producing reaction:
Use of ferric ion as electron acceptor
The ferric ion (Fe 3+ ) can be used by various bacteria as an electron acceptor, reducing it to a ferrous ion (Fe 2+ ); This process is carried out by many of the microorganisms that reduce nitrate. The ferric ion is found in soil and rocks, often forming insoluble ferric hydroxide (Fe (OH) 3 ); Under anaerobic conditions, these bacteria can reduce it to the ferrous state. The ferrous ion is more soluble than the ferric ion, with which the iron is mobilized, this being an important first step in the formation of a type of mineral deposit called swamp iron.
-
Glycolysis
Glycolysis is the metabolic pathway that allows glucose energy to be obtained . In other words, it is a successive series of biochemical reactions, applied by most living things , to break the glucose molecule (C 6 H 12 O 6 ) and obtain the necessary chemical energy (in the form of ATP ) to keep the cellular metabolism going .
Glycolysis consists of 10 enzymatic reactions that occur consecutively, either in the presence (aerobic) or in the absence (anaerobic) of oxygen . It results in the formation of two molecules of pyruvate or pyruvic acid (C 3 H 4 O 3 ), which feed other metabolic pathways to continue obtaining energy for the organism (the so-called Krebs Cycle).





