Difference between groups and periods in tabular form
 We explain that what is the difference between period and group with a table. The difference between periods and groups is their arrangement, periods are arranged horizontally, while groups are arranged vertically on the periodic table.
We explain that what is the difference between period and group with a table. The difference between periods and groups is their arrangement, periods are arranged horizontally, while groups are arranged vertically on the periodic table.
The periodic table of chemistry is something that students are asked to tediously memorize without even knowing the real reason behind learning it. For some people, it is just a topic that is included in their syllabus. But in a real sense, this little periodic table is much more important than that, it is a roadmap that opens a million opportunities for scientists and researchers around the world.
Dmitri Mendeleyev is the inventor of the periodic table. Before him, many made efforts to arrange the chemical elements in different ways. But Dmitri’s result was accepted around the world.
In 1869, Dmitri Mendeleyev disseminated a table of elements, on the basis of which the new periodic table was subsequently built. Therefore, all credit goes to Mendeleyev for his contributions to creating a portal for the enlightening new periodic table of elements.
When you view the periodic table, you will notice that there are row headings (left to right) and columns running (top to bottom).
In scientific abbreviations, the rows are called Periods and the columns are Groups, respectively.
Mendeleyev during the time of organization left some empty rows with the impression that there would be other elements that would enter in the near future. And surprisingly, one of the elements that fit in that space was Galio.
There are many interesting facts about the search for elements of the periodic table, but precisely here we would concentrate more on the periods and the arrangement of the columns.
The common thing between these two is that the atomic number increases both vertically and horizontally.
Comparison table between period and group
Comparison period group parameter
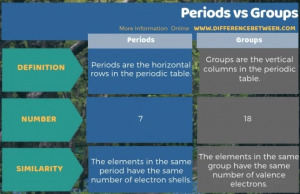
| Direction | Periods are the horizontal rows of the modern periodic table | The groups are the vertical columns that run across the top of the periodic table. |
| Properties | The elements of a period do not have similar properties. | The elements of each group have some similar properties, but not totally identical. |
| Likeness | Elements in the same period have the same number of electron composition | The elements in each group have the same number of valence electrons. |
| Sum | There are 7 periods in the periodic table | The group contains 18 elements arranged vertically in the modern periodic table. |
| Electronegativity | Increase from left to right. | Increase from bottom to top in a group. |
How are periods and groups different from each other?
What is the difference between a period and a group family?
What is the period?
A period is a horizontal row that runs from the extreme left to the extreme right in the periodic table. As of now, there are 7 periods on the periodic table. A new period begins when a new fundamental energy level is added to the electrons.
Each element of a period is likely to have the same number of atomic orbitals.
For example, each element in the first period has only 1 orbital for its own electrons, the second period includes 2 orbitals for electrons. Likewise, as you move down the row, the orbitals keep adding.
The size of the element decreases as it progresses through a period, since the number of electron shells remains constant during the period, but the number of protons increases in the nucleus. This is the reason why the atom gets heavier but the size keeps decreasing.
If you look at the periodic table, you will see that there are different elements nested in each row.
The first period has only 2 elements (1 and 18), the second and third periods have 8 elements each, the fourth and fifth periods have 18 elements, and finally, the sixth and seventh periods have 32 elements each, respectively.
What is the group?
When you count from top to bottom, there are a total of 18 groups on the periodic table. All groups are assigned different names.
Groups are mixed categories of metals, nonmetals, and semimetals that are grouped into families according to their similar properties. For example, Group 1 belongs to the lithium family classified as alkene metals. Similarly, each group in the lane has its own last name.
The elements of the related group have similar traits because they have the same electron count in their outermost shell.
The size of the item increases as it moves down in any group. This is because there are a large number of protons and neutrons in the nucleus. In addition to this, an extra electron shell is what makes the atom heavier.
For groups, there are two different ways to illustrate the items. It is important to understand both numbering systems because the periodic table appears in both formats.
In the United States, they used the letters A and B to indicate each item in the group, but unfortunately, it was observed as a disorganized numbering system.
To eliminate any possible confusion, the International Union of Pure and Applied Chemistry (IUPAC) came up with the idea of numbering the elements as (1,2, 3… 18). However, both numbering systems are acceptable. But the IUPAC numbering seems simple and well organized.
Main differences between Period and group
- Location: The groups are the vertical column, while the periods are the straight rows of the periodic table.
- Number: There are a total of 18 groups and 7 periods in the periodic table among which the groups are classified into different families and types of metals.
- Chemical properties: All the elements of the group have analogous chemical or physical properties, while the periods share the same electronic hierarchy.
- Energy level: As we go down the group from top to bottom, the energy level of the electron increases. On the other hand, in each period the energy level of the electron remains the same.
- Electronegativity: This decreases from top to bottom in a group and increases from left to right in a period. This is an important consideration when studying the periodic table.
Final Thought
Groups and periods are the two ways to classify chemically organic or inorganic elements or metals present in the modern periodic table and the key difference between group and period is their location on the periodic table. As you already know, the points are on the horizontal line and the groups are on the vertical line.


