What is thermal conductivity?
We explain what thermal conductivity is and the methods used by this property. In addition, its units of measure and examples.
-
What is thermal conductivity?
There is talk of thermal conductivity to refer to a property of certain materials capable of transmitting heat , that is, allowing the passage of the kinetic energy of its molecules to other adjacent substances. It is an intensive magnitude, inverse to the thermal resistivity, which is, logically, the resistance of certain materials to the transmission of heat by their molecules.
The explanation of this phenomenon is that, when a material is heated, its molecules receive an extra kinetic energy that increases its agitation. The molecules, then, are able to share that extra energy without causing global movements of matter (in that it differs from the thermal convection of liquids and gases ), this capacity being very high in metals and in continuous bodies, Usually, and very low in polymers and other insulating materials such as fiberglass.
Thus, the thermal conductivity of a material is calculated from a coefficient (referred to as λ) and is different depending on the molecular nature of each specific material. This calculation is made based on the following formula:
λ = [Equation] / [Equation]
Where ‘q is the heat flux per unit of time and area, and [Equation] is the temperature gradient. The higher the thermal conductivity of a material, the better heat conductor will result , and the lower that one is, the material will be more insulating. Temperature, convection, electrical conductivity and phase changes of the material all influence the result of the coefficient of thermal conductivity.
-
Thermal conduction methods
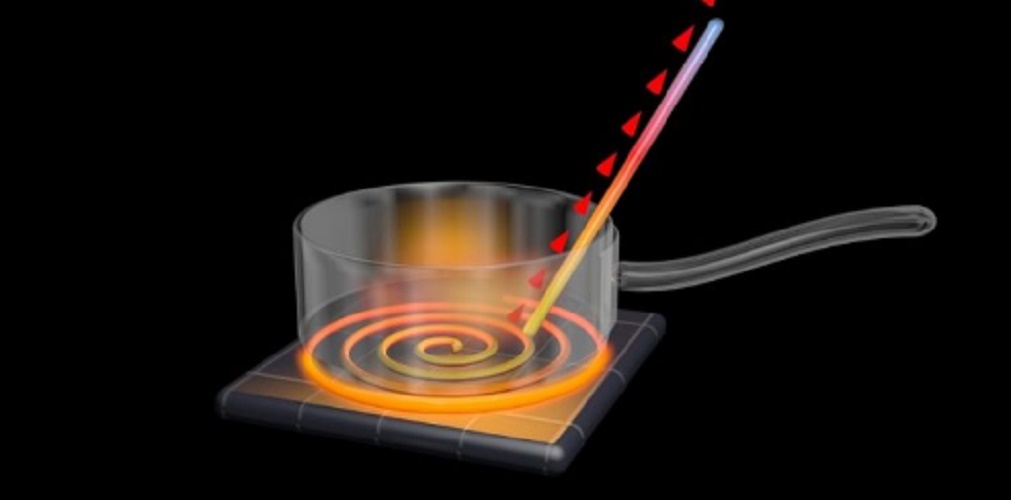
There are three methods of heat transmission in nature: conduction, convection and radiation.
- Conduction : occurs when heat is transmitted from one body to another at a different temperature through mere contact, without a displacement of matter.
- Convection : It is produced through the movement of particles of the substance that transmits heat, so it must always be a fluid (liquid or gas), either by natural or forced movement .
- Radiation : Occurs when heat is transmitted between two solids of different temperatures without any contact point or conductive solid between them. Heat is transmitted in the emission of electromagnetic waves at the speed of light .
-
Units of measurement of thermal conductivity
Thermal conduction is measured, according to the International System, from the ratio W / (Km), which is equivalent in units to Joules per meter per second per Kelvin (J / msK).
Thus, a thermal conductivity of 1 watt per meter and Kelvin means that a July (J) of heat is propagated through a surface material of 1m2 and a thickness of 1m, in 1 second, when the difference between both substances is 1K
-
Examples of thermal conductivity
Some examples of thermal conductivity are:
- Steel , with a conductivity of 47 to 58 W / (Km).
- Water , with a conductivity of 0.58 W / (Km).
- Alcohol , with a conductivity of 0.16 W / (Km).
- The bronze , with a conductivity of 116 to 140 W / (Km).
- Wood , with a conductivity of 0.13 W / (Km).
- Titanium , with a conductivity of 21.9 W / (Km).
- Mercury , with a conductivity of 83.7 W / (Km).
- Glycerin , with a conductivity of 0.29 W / (Km).
- The cork , with a conductivity of 0.03 to 0.04 W / (Km).
- Gold , with a conductivity of 308.2 W / (Km).
- Lead , with a conductivity of 35 W / (Km).
- The diamond , with a conductivity of 2300 W / (Km).
- The glass , with a conductivity of 0.6 to 1.0 W / (Km).
- Lithium , with a conductivity of 301.2 W / (Km).
- The wet earth , with a conductivity of 0.8 W / (Km).





